By about 1815 water closets of this type had become common in london households. List grid map price.
A water closet is a room that contains a flush toilet usually accompanied by a washbowl or sink and the term may also be used to refer specifically to a flush toilet.
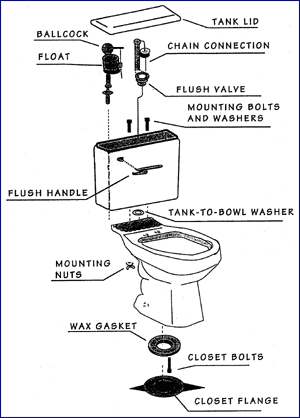
Parts of water closet. The holes under the lip of the toilet seat. Flapper tank ball the lift chain operates a rubber flapper that rests against the flush valve opening. Siphon jet water closet parts siphon jet water closet parts.
Parts of water closet. February 10 2020 lukman foto 0. How a toilet works toilet seat cover marinespares parts of a toilet the parts of a toilet the four types of toilets.
Speakers of british english may refer to such a room as a w c referencing the initials for this term. The bramah water closet patented by joseph bramah in 1778 used a similar but more complex flushing device that kept the water running for about 15 seconds. There is a minimum advertised price map allowed by the manufacturer on this product.
Parts unless otherwise indicated. Understanding the parts of a toilet. A ballcock also balltap or float valve is a mechanism or machine for filling water tanks such as those found in flush toilets while avoiding overflow and in the event of low water pressure backflow.
Ring of wax found between the flange and bottom of the toilet. The many parts of a toilet 3 diagrams tank plumbing. Universal fit toilet flapper with chain and hook.
A wide variety of water closet toilet parts options are available to you such as graphic design 3d model design and others. Parts of water closet toilet. The u shaped part of the pipe that holds water and prevents smelly gas from rising to the toilet.
The development of. Also fits crane toilets the reference of manufacturer s names is for reference only and does not indicate o e m. A flush toilet also known as a flushing toilet water closet wc see also toilet names is a toilet that disposes of human waste urine and feces by using water to flush it through a drainpipe to another location for disposal thus maintaining a separation between humans and their waste.
Safety feature used to prevent water from exiting the toilet tank. In older toilets this may be a tank ball instead you may not be able to clearly see the flapper with water in the tank but its operation will become clear when you flush the toilet and watch the action. Flush toilets can be designed for sitting in which case they are also called western toilets or.
At the lower atmospheric pressure on the top of Mount Everest pure water boils at about 154 F 68C. This is also equivalent to 100 degrees Celsius.
 Question Video Knowing The Boiling Point Of Water Nagwa
Question Video Knowing The Boiling Point Of Water Nagwa
This means it takes pasta and rice longer to get done and you may need to add more water to the pot as it boils off before they are the right consistency.

Water boils in celsius. Liquids boil when the pressure of the atmosphere is equal to the pressure of the liquid. The boiling point of water is dependant on pressure. The temperature scale was developed in 1734 by German physicist Daniel Gabriel Fahrenheit who also invented the first modern thermometer.
The boiling point of water is 37315 degrees Kelvin K. In the lab atmospheric pressure changes due to. When the pressure of the atmosphere is reduced a liquid boils at a lower temperature.
Water boils at 100 C or 212 F at one atmosphere of pressure. Before I get to the proper definition let us study boiling according to the widely accepted definition. Click to enlarge.
Though its one of the basic facts you probably learnt pretty early on back in school science lessons your elevation relative to sea level can affect the temperature at which water boils due to differences in air pressure. The celsius scale was originally defined around the boiling 100oC and freezing point 0oC of water and hence the scale was defined by waters boiling. The boiling point of water in Celsius Fahrenheit and Kelvin scales are 100 degree Celsius or C 212 degree Fahrenheit or F and 373 Kelvin or K.
However this is not a coincidence. So we say that at the temperature at which water forms bubbles it is boiling. Your pot of water will come to a boil sooner as it will boil at a lower temperature than at sea level.
You can boil water at about 50 C in this system. Water boils at 100 degrees Celsius. If we go according to this definition then water definitely boils at 100 degrees Celsius.
Water boils at 100 C or 212 F at one atmosphere of pressure. Fahrenheit temperature scale scale based on 32 for the freezing point of water and 212 for the boiling point of water the interval between the two being divided into 180 equal. At sea level pure water boils at 212 F 100C.
You can boil water at about 50 C in this system. Given the temperature in one scale we can. Liquids boil when the pressure of the atmosphere is equal to the pressure of the liquid.
The boiling point of water varies with atmospheric pressure. However this definition is not acceptable in the big picture. The explanation goes back to the definition of the boiling point of water which is that the water boils at 100 degrees Celsius at one atmospheric pressure because at one atmospheric pressure vapor pressure of vapors above water becomes equal to atmospheric pressure now as you create a vacuum inside the syringe you reduce atmospheric pressure significantly and much fewer vapors or.
At lower pressure or higher altitudes the boiling point is lower. Pure water boils at 100 degrees celcius at standard atmospheric pressure. You would boil your food longer because it is boiling at a lower temperature.
Using the Fahrenheit scale waters boiling point is 212 degrees. Pure water boils at 100 degrees Celsius under normal atmospheric pressure but the Dead Sea surface is so low more than 400 meters below sea level that water boilsat about 101 degrees there. When the pressure of the atmosphere is reduced a liquid boils at a lower temperature.
1At what temperature does water boil in degrees celsius. Water always boils at 100C right. We all learn at school that pure water always boils at 100C 212F under normal atmospheric pressure.
And removing dissolved air from water can easily raise its boiling temperature by about 10 degrees centigrade.
12 Cons of Purified Water. Just because distilled water happens to be purer than other kinds of water doesnt make it healthier to drink.
 Distilled Water Vs Purified Water
Distilled Water Vs Purified Water
The article explained what purified water is and distilled water its pros and cons and the differences.
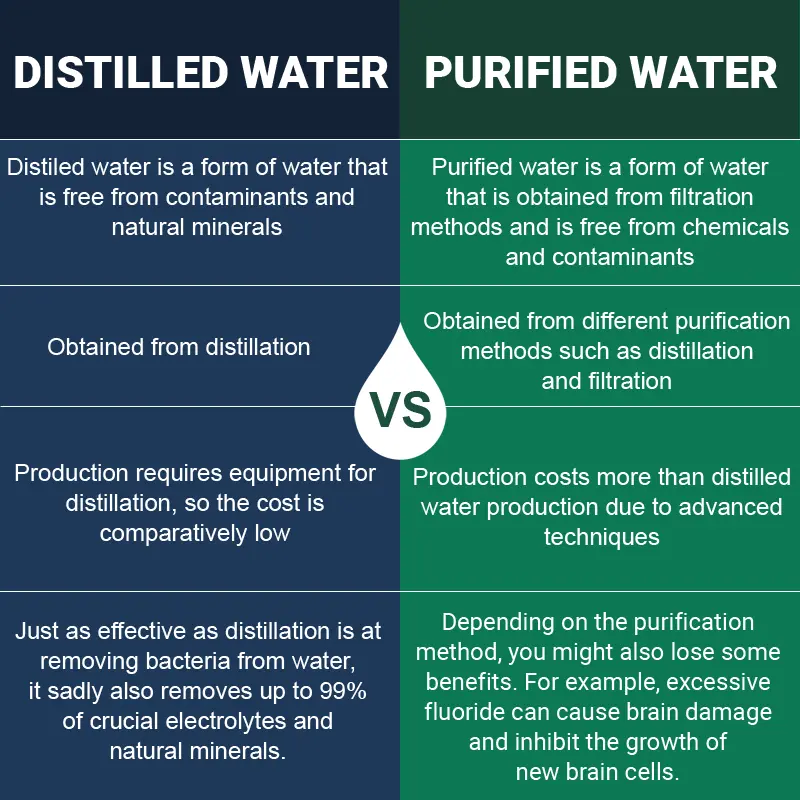
Purified vs distilled water. What is Purified Water. The distillation process removes fluoride and natural minerals found in. Also distilled water is the closest thing you can get to the purest water with less than 05 impurities.
Why is it important to have an alkaline bodyAlkalizing is a necessity to eliminate the probability of diseaseReduces inflammation. It does not dissolve solid as distilled do but it is still known as healthy water that can be used cooking. The bottom line is that any type of water that has been filtered and purified in some way can be called purified water.
This means that distilled water is a type of purified water. Purified water on the other hand gets processed via other means such as sand filtration reverse osmosis and. A single gallon of distilled water is produced in 4-6 hours using this method.
Pros and cons of purified water. 21 Health Benefits of Distilled Water. Distilled water is a form of purified water that is purified through a complex boiling process.
11 Health Benefits of Purified Water. Purified vs distilled water. So we have come to the conclusion that distilled water is purified water that uses the distillation method out of many others.
Over-acidity in the bo. On the other hand purified water. The biggest difference between distilled water and purified water is the method used to produce the end result.
Distilled water is always 100 pure but purified water might still contain tiny traces of impurities. Distilled water and purified water are the two options that people want to try over tap water. Purified water just like spring water has several advantages and drawbacks.
Distilled Water VS Purified Water Whats the When To Drink Protein Shakes For Weight How To Store Potatoes Long Term without How To Grow Taller Overnight And Other How Fast Does Hair Grow Men New Foods That Boost Testosterone. The high purity level of distilled water comes at a price because while the distillation process removes almost 100 of contaminants it also removes beneficial nutrients such as electrolytes and minerals. Distilled water might be good for the short term but you would better not use it for the long term as it doesnt hold the required minerals.
The water is then stored as pure distilled water. It can be created through several processes such as reverse osmosis and distillation. People who want clean water that still includes minerals choose purified water.
How To Increase Walk Behind Brush Cutter Rental Home Depot How To Use Protein Powder For. Purified water must contain less than 10 parts per million of dissolved solids. The only minor difference between purified and distilled water is that boiling water in distillation utilizes a high amount of energy to transform the liquid into steam.
Whats the Difference Between Purified vs. Purified water is pure than filtered water. Less exposure to hazardous substances traces of prescription medications and other dangerous compounds.
As you may gather from the information above there is essentially no difference between purified and distilled water aside from the purification process. 22 Cons of Distilled Water. Distilled water goes through distillation while purified water is processed by other means reverse osmosis sand filtration ion exchange etc.
While purified water is made through various methods of filtration distilled water is made in an age-old process of distillation that has become modernized to what it is today. All impurities in the water inclusive of ions minerals and nitrates are left behind in the original container. Distilled water is a type of purified water that is essentially free from contaminants.
Purified water is as free from the same bacterial and sponging pollution as distilled water is. Those who need water that is sterile and completely bacteria free turn to distilled water. The difference and which is best for health Written by Nadine Brooks on January 18 2021 The cleanliness and healthful properties of water remain an important concern.
In very simple terms and according to its dictionary definition purified water is water which has gone through some sort of purification process. The water is vaporized until it turns to steam the steam is transported to a clean container and then condensed back into a liquid. Distilled water is free of all impurities which makes the mineral content zero.
Distilled water is created by collecting steam from boiling water. 41 Health Benefits of Purified Water.
610 m 208 ºF. At altitude water boils at 934 C 2001 F at 1905 meters 6250 ft.
/BoilingWater-58dd1c2a5f9b5846837d2a23.jpg) What Is The Boiling Point Of Water
What Is The Boiling Point Of Water
The boiling point for water at sea level and under standard conditions is 100 degrees Celsius 212F.
Boiling water in celsius. 457 m 209 ºF. Does water freeze faster at higher altitude. British Standard 6008 and International Standard ISO 3103 advise that tea is best made with water that is freshly boiled.
The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. What is the Boiling Point of Water. 100 - the boiling point of water.
1067 m 2055 ºF. However all waterborne intestinal pathogens are killed above 60 degrees Celsius. Pure water boils at 100 degrees Celsius under normal atmospheric pressure but the Dead Sea surface is so low more than 400 meters below sea level that water boilsat about 101 degrees there.
It does boil at a higher temperature because of the high amounts of non-volatile solids. Can you make water boil at 70 C. Find your local pressure and elevation.
1000 ft 305 m 210 ºF. Water boils at 3732 K Kelvin 100ºC Celsius or 212ºF Fahrenheit. It boils at 100 Celsius at sea level and a little bit below it when you get higher but the difference isnt that much.
Boiling Point - Celsius. 762 m 207 ºF. To use this calculator you will need your current pressure and elevation.
When measuring temperature the usual units are Celsius degree Celsius or Fahrenheit degree Fahrenheit. In the lab atmospheric pressure changes due to the weather and height above sea level. When you get your water to 75 Celsius you are heating it not boiling it.
The explanation goes back to the definition of the boiling point of water which is that the water boils at 100 degrees Celsius at one atmospheric pressure because at one atmospheric pressure vapor pressure of vapors above water becomes equal to atmospheric pressure now as you create a vacuum inside the syringe you reduce atmospheric pressure significantly and. Even in the highest towns in the world at more than 5000 meters water boils at just below 90 Celsius. 0 m 212 ºF.
Pure water boils at 100 degrees celcius at standard atmospheric pressure. 152 m 211 ºF. Temperature given as C F K and R.
However the value is not a constant. Water boils at 212F at sea level but only at sea level. This puts the boiling and freezing points of water 180 degrees apart.
Also what temperature does water boil in Celsius and Fahrenheit. The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. However when reporting temperatures in Kelvin we dont say degree Kelvin.
How to Calculate the Boiling Point of Water. 50 degree Celsius water has vapor pressure of 123 mbarYou can make the water boiling by making the ambient pressure lowA 60mL syringe can create pressure. Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara.
The boiling point of water is 37315 degrees Kelvin K. Since the Water boils at 100 Celsius at level of the sea. 1219 m 204 ºF.
Clostridium bacteria can survive in boiling water even at 100 degrees Celsius which is its boiling point for several minutes. Click to see full answer. Yes You can make water boil at 70 C.
It was redefined in 1954 to be based on absolute zero and the triple point of water which related the Celsius scale precisely to the Kelvin scale. Using the Fahrenheit scale waters boiling point is 212 degrees. 3000 ft 914 m 206 ºF.
The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure sea level. Reduce the atmospheric pressure around the water by using a vacuum pump and a closed container else go into a very high altitude. The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F.
On the Fahrenheit scale the freezing point of water is 32 degrees Fahrenheit F and the boiling point is 212 F at standard atmospheric pressure. Changes in atmospheric pressure will alter the temperature at which water boils. The boiling point of water depends on the atmospheric pressure which changes according to elevation.
This is also equivalent to 100 degrees Celsius. This allows the water to boil at a lower temperature and many more liquids too. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius but only at sea level.
The freezing and boiling points of water were part of the definition of the Celsius scale for a long period of time 1743-1954. The boiling point of water is dependant on pressure. Boiling Point - Fahrenheit.
This is because its spores can withstand temperatures of 100 degrees Celsius. 51 views Answer requested by. Please share our efforts.