Alkaline-earth metal - any of the bivalent metals of group II of the periodic table calcium or strontium or barium or magnesium or beryllium alkaline earth metal metallic element - any of several chemical elements that are usually shiny solids that conduct heat or electricity and can be formed into sheets etc. Both alkali metals and alkaline earth metals are good electrical and heat conductors.
Any of the chemical elements beryllium magnesium calcium strontium barium and radium.
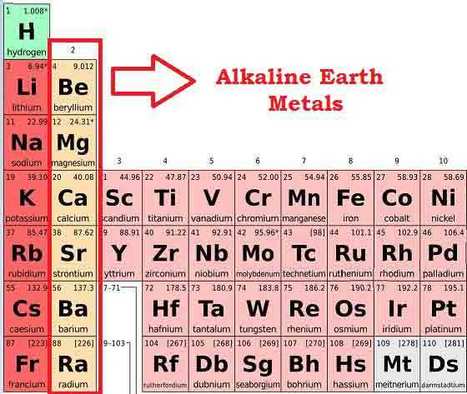
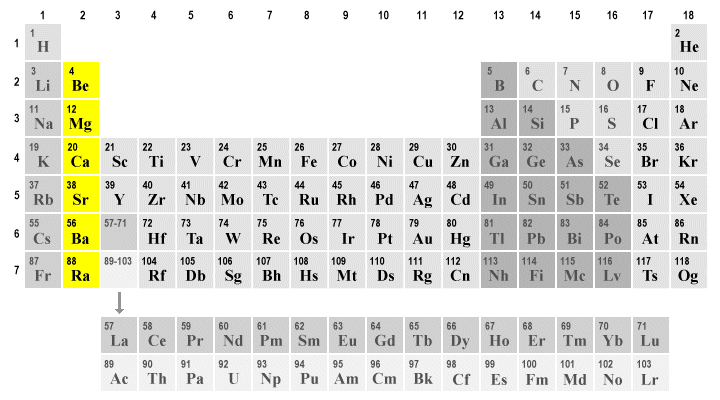
Definition of alkali earth metals. The elements highlighted in yellow on the periodic table in the graphic belong to the alkaline earth element group. Although they are soft enough to cut with a knife exposing a bright surface the metals react with water and air and quickly tarnish so the pure metals are stored in an inert atmosphere or under oil to prevent oxidation. Any of the divalent strongly basic metals of group II of the periodic table comprising beryllium magnesium calcium strontium barium and radium.
Alkali metals are all soft shiny reactive metals. Alkali or alkali earth metalcompounds in which for instance the anion is a polymeric sulphonated phenol are patented as deposit control agents. Here is a look at the location and the properties of these elements.
Alkaline-earth metal any of the six chemical elements that comprise Group 2 IIa of the periodic table. The most essential compounds as fuel catalysts are alkali or alkali earth metalderivatives especially potassium salts of organic acids soluble in petrol. The alkaline earth metals are one group of elements on the periodic table.
Alkaline earth metal meaning. Definition of alkali metal. Definition of alkaline earth metal.
Alkaline earth metals definition roles of alkali alkaline earth metals alkali metal definition chemistry alkaline earth metals definition properties of the alkaline earth metals. Any of the bivalent metals of group II of the periodic table calcium or strontium or barium or magnesium or beryllium. Location of the Alkaline Earths on the Periodic Table.
Alkali metals and alkaline earth metals are the S-block elements because elements in both of these groups have their outermost electrons in the s-subshell. Any of the monovalent mostly basic metals of group I of the periodic table comprising lithium sodium potassium rubidium cesium and francium see Periodic Table Examples of alkali metal in a Sentence. The alkaline earth metals are a group of chemical elements in the periodic table with very similar properties.
See Periodic Table. The elements in these two groups are the most reactive metals in the periodic table. Usually calcium strontium magnesium and.
Alkaline earth metal definition. The elements are beryllium Be magnesium Mg calcium Ca strontium Sr barium Ba and radium Ra. Called also alkaline earth.
Any of the chemical elements beryllium magnesium calcium strontium barium and radium. Alkaline Earth Metals Definition Location In. They are all shiny silvery-white somewhat reactive metals at standard temperature and pressure and readily lose their two outermost electrons to.
Alkali And Alkaline Earth Metals. Definition of alkaline-earth metals - Chemistry Dictionary Definition of alkaline-earth metals the heaviest members of the group IIa in the periodic table.
Alkaline Earth Metals The Periodic Table
 Alkaline Earth Metals Definition Location In The Periodic Table
Alkaline Earth Metals Definition Location In The Periodic Table
Definition Of Alkaline Earth Metals Video Of Magnesium
 Alkali Metal Definition Properties Facts Britannica
Alkali Metal Definition Properties Facts Britannica
The Msds Hyperglossary Alkaline Earth
 Alkali And Alkaline Earth Metals
Alkali And Alkaline Earth Metals
 Group 2 Elements Alkaline Earth Metals Emedicalprep
Group 2 Elements Alkaline Earth Metals Emedicalprep
/alkalineearth-56a12cd75f9b58b7d0bcca7e.png)